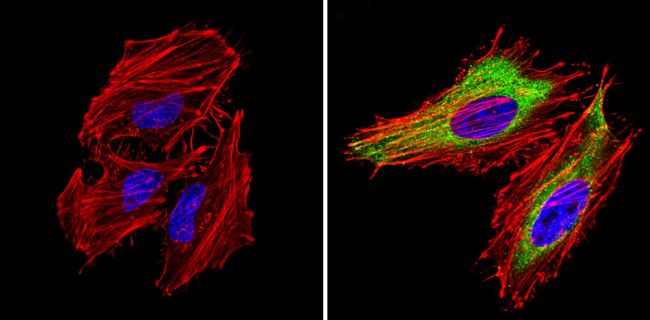
CXCR4 Polyclonal Antibody for Western Blot, IF, ICC, IHC (P) and Flow
日本天野酶试剂官网
日本Amano酶中国官网代理商

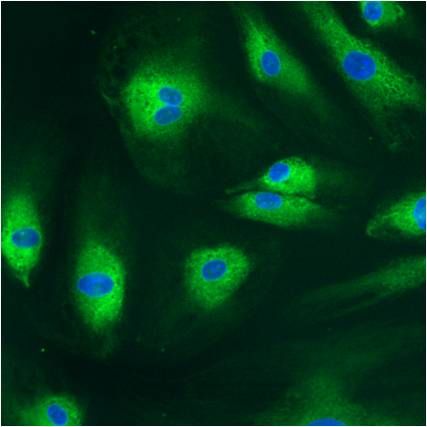
CXCR4 Antibody (PA3-305) in IF
Immunofluorescent analysis of CXCR4 (green) showing staining in the cytoplasm of Hela cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a CXCR4 polyclonal antibody (Product # PA3-305) in 3% BSA-PBS at a dilution of 1:100 and incubated overnight at 4°C in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. Actin was stained using Alexa Fluor 554 (red) and nuclei were stained with Hoechst or DAPI (blue). Images were taken at a magnification of 60x.
HDAC2 Antibody (51-5100) in IF
Immunofluorescence analysis of HDAC2 was done on 70% confluent log phase A431 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with HDAC2 Rabbit Polyclonal Antibody (515100) at 2ug/ml in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing nuclear localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.

ZO-1 Antibody (61-7300) in IF
Immunofluorescent analysis of ZO-1 (green) in Caco-2 cells. The cells were fixed with 4% paraformaldehyde for 15 minutes and blocked with 3% Blocker BSA (Product # 37525) in PBS for 15 minutes at room temperature. Cells were stained with or without ZO-1 rabbit polyclonal antibody (Product # 61-7300), at a concentration of 5ug/ml for 1 hour at room temperature, and then incubated with a Goat anti-Rabbit IgG (H+L) Superclonal Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:1000 for 1 hour at room temperature (both panels, green). Nuclei (both panels, blue) were stained with Hoechst 33342 dye (Product # 62249). Images were taken on a Thermo Scientific ToxInsight at 20X magnification.

Glucocorticoid Receptor alpha Antibody (PA1-516) in IF
Immunofluorescent analysis of Glucocorticoid Receptor alpha using Glucocorticoid Receptor alpha Polyclonal Antibody (Product# PA1-516 ) shows staining in A2058 Cells. Glucocorticoid Receptor alpha (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with an antibody recognizing Glucocorticoid Receptor alpha (Product# PA1-516 ) at a dilution of 1:200 over night at 4 °C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product# 35552 for GAR, Product# 35503 for GAM). Images were taken at 60X magnification.

NFkB p65 Antibody (PA1-186) in IF
Immunofluorescence analysis of NF-kB p65 was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with NF-kB p65 Rabbit Polyclonal Antibody (PA1186) at 1ug/mL in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing cytoplasmic and nuclear localization. Panel e is a no primary antibody control. The images were captured at 60X magnification.

Phospho-SMAD2 (Ser465, Ser467) Antibody (44-244G) in IF
Immunofluorescence analysis of Phospho-SMAD2 pSer465 / pSer467 Antibody was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were treated with TGF-beta1(20ng/mL) for 15 minutes and labelled with Phospho-SMAD2 pSer465 / pSer467 Antibody(44244G) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa flour 488 Goat Anti-Rabbit IgG Secondary Antibody (A11008) at a dilution of 1:400 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (A12381). Panel d is a merged image showing Nuclear localization. Panel e showing merged image of untreated cells with less nuclear signals Panel f is a no primary antibody control. The images were captured at 40X magnification.

GATA4 Antibody (PA1-102) in IF
Immunofluorescent analysis of GATA4 (green) in embryoid body endoderm generated from Gibco ® Human Episomal iPSC Line grown on Geltrex® in Essential 8TM Medium. After 2 weeks in culture, EB were dissociated with TrypLETM and re-plated onto Geltrex®-coated multi-well plates. Cells were fixed, permeabilized and blocked for immunostaining. Cells were stained with a GATA4 polyclonal antibody (Product # PA1-102) at a dilution of 1:100 in 3% BSA/PBS blocking buffer overnight at 4°C, and then incubated with Alexa Fluor® 488 donkey anti rabbit antibody (Product # A21206) at a 1:500 dilution in conjunction with NucBlue® Fixed Cell Ready Probes® Reagent. After another 3 washes, images were taken on EVOS®Floid® Cell Imaging system at 10X magnification.

alpha Tubulin Antibody (A11126) in IF
Immunofluorescence analysis of Alpha-Tubulin was done on 70% confluent log phase HeLa cells. The cells were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.25% Triton™ X-100 for 10 minutes, and blocked with 5% BSA for 1 hour at room temperature. The cells were labeled with Alpha-Tubulin Mouse monoclonal Antibody (A11126) at 1µg/mL in 1% BSA and incubated for 3 hours at room temperature and then labeled with Alexa Flour 488 Rabbit Anti-Mouse IgG Secondary Antibody (A11059) at a dilution of 1:400 for 30 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant DAPI (S36938). F-actin (Panel c: red) was stained with Alexa Fluor 594 Phalloidin (A12381). Panel d is a merged image showing cytoplasmic localization. Panel e shows no primary antibody control. The images were captured at 20X magnification.

LIN28A Antibody (PA1-096) in IF
Immunofluorescent analysis of LIN28 (green) in Hela cells. Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes at room temperature and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a LIN28 polyclonal antibody (Product # PA1-096 ) at a dilution of 1:100 and incubated overnight in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody for 45 minutes at room temperature in the dark. F-actin (red) was stained with a fluorescent phalloidin and nuclei (blue) were stained with DAPI. Images were taken at a 60X magnification.

PDI Antibody (MA3-019) in IF
Immunofluorescent analysis of Phalloidin (orange) and PDI (green) in NIH 3T3 cells. Formalin fixed cells were permeabilized with 0.1% Triton X-100 in PBS for 10 minutes at room temperature and blocked with 2% BSA (Product # 37525) in PBS + 0.1% Triton X-100 for 30 minutes at room temperature. Cells were probed with a PDI monoclonal antibody (Product # MA3-019) at a dilution of 1:75 for at least 1 hour at room temperature, washed with PBS, and incubated with DyLight 488 goat anti-mouse IgG secondary antibody (Product # 35502) at a dilution of 1:250 for 30 minutes at room temperature. Actin was stained with DyLight 550 Phalloidin (Product # 21835) at a dilution of 1:120 (2.5 units/ml final concentration) and nuclei (blue) were stained with Hoechst (Product # 62249) at a concentration of 1ug/ml for 30 minutes. Images were taken on a Zeiss Axio Observer Z1 microscope at 20X magnification.