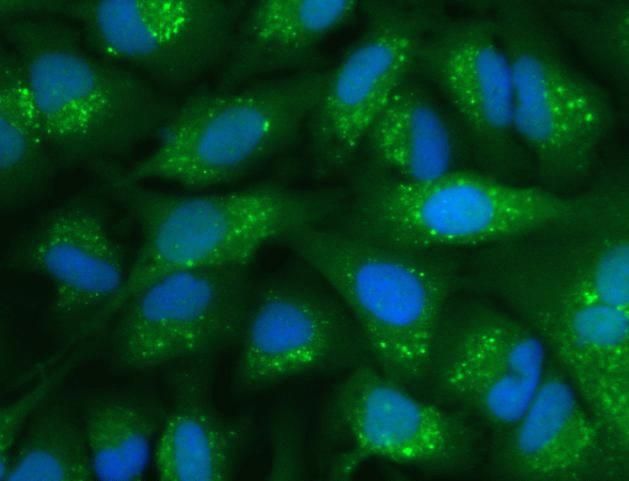
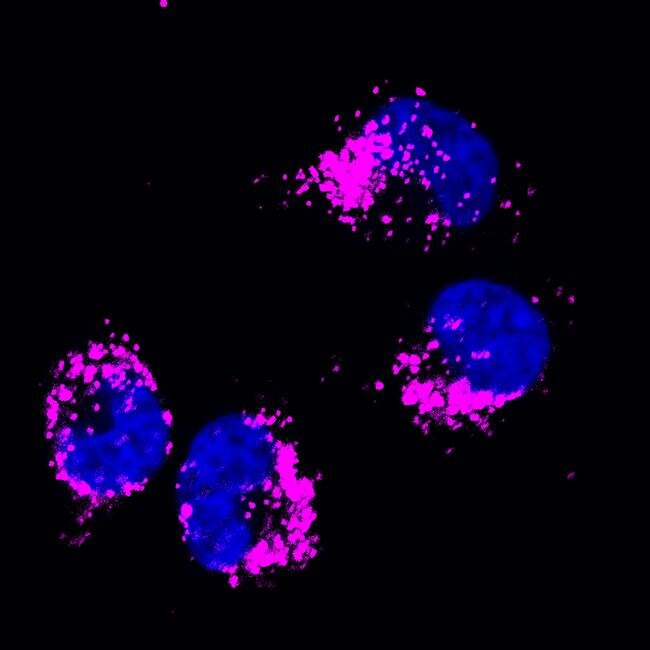
HSC70 Monoclonal Antibody for Western Blot, IF, ICC, IHC (F), Flow and IP
日本天野酶试剂官网
日本Amano酶中国官网代理商


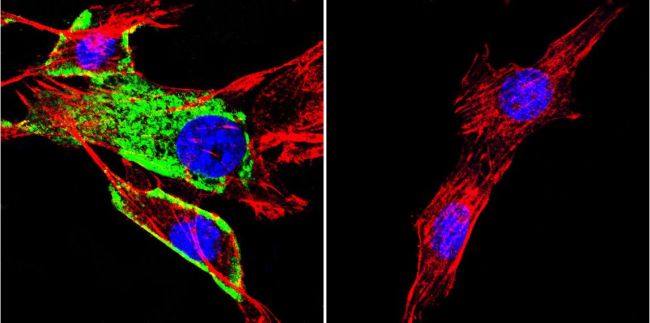
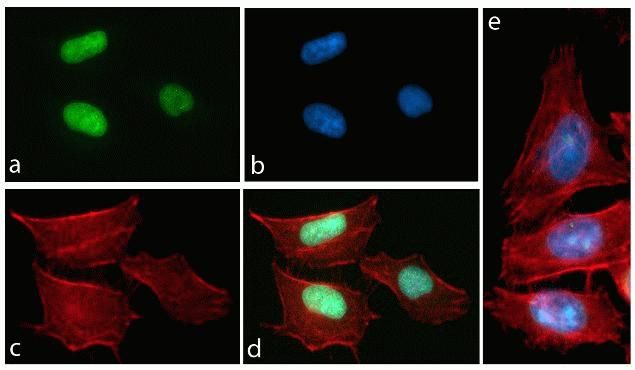
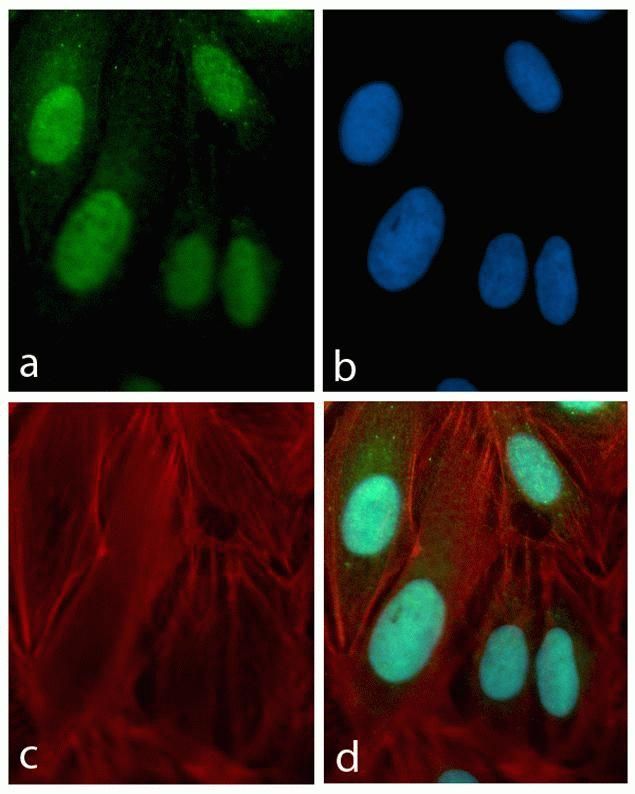
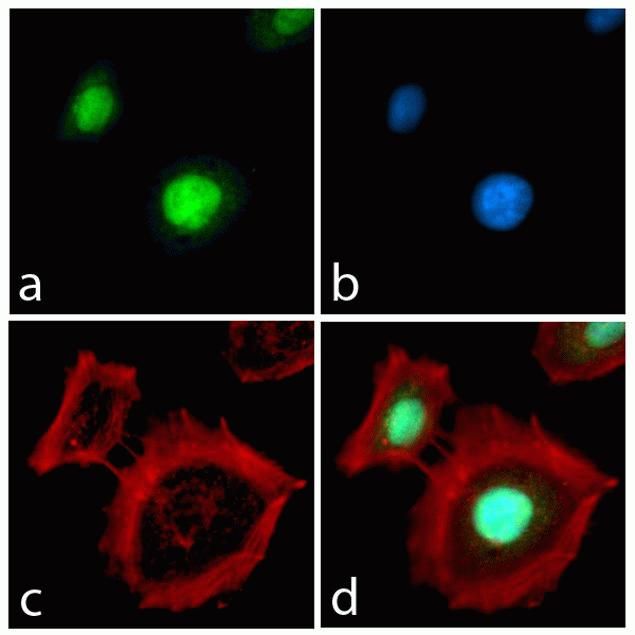
HSC70 Antibody (MA3-014) in IF
Immunofluorescence analysis of HSC70 / Heat Shock Cognate Protein was performed using 70% confluent log phase Caco-2 cells. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with HSC70 / Heat Shock Cognate Protein Mouse Monoclonal Antibody (MA3014) at 1:250 dilution in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Mouse IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (A28175) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (S36938). F-actin (Panel c: red) was stained with Rhodamine Phalloidin (Product # R415, 1:300). Panel d represents the merged image showing cytoplasmic and membranous localization. Panel e shows the no primary antibody control. The images were captured at 60X magnification.

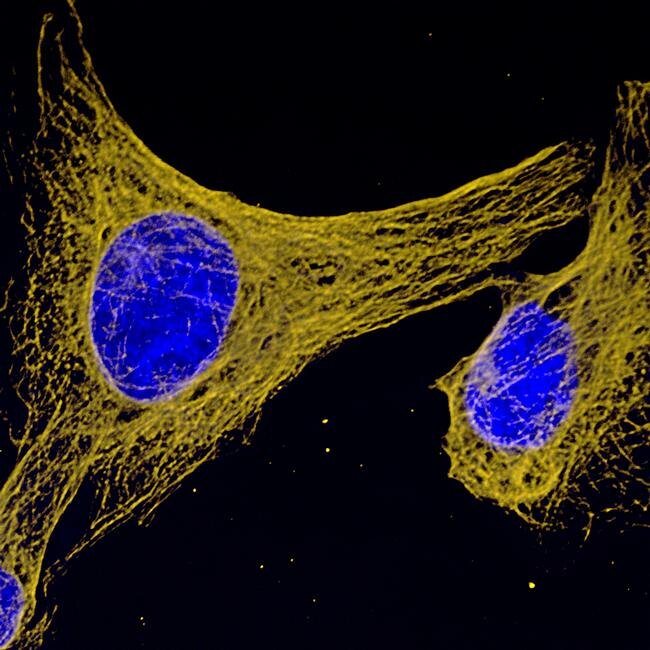
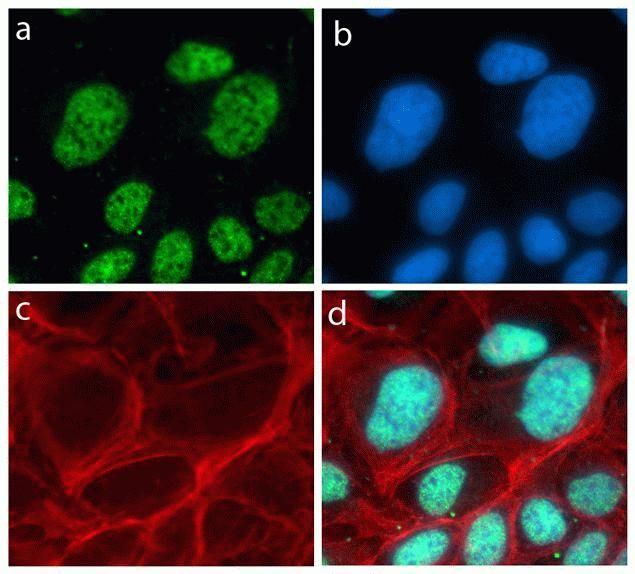
Cyclin B1 Antibody (MA1-155) in IF
Immunofluorescent analysis of Cyclin B1 (green) showing staining in the in the cytoplasm and nucleus of C2C12 cells (right) compared to a negative control without primary antibody (left). Formalin-fixed cells were permeabilized with 0.1% Triton X-100 in TBS for 5-10 minutes and blocked with 3% BSA-PBS for 30 minutes at room temperature. Cells were probed with a Cyclin B1 monoclonal antibody (Product # MA1-155) in 3% BSA-PBS at a dilution of 1:150 and incubated overnight at 4°C in a humidified chamber. Cells were washed with PBST and incubated with a DyLight-conjugated secondary antibody in PBS at room temperature in the dark. F-actin (red) was stained with a flourescent red phalloidin and nuclei (blue) were stained with Hoechst or DAPI. Images were taken at a magnification of 60x.

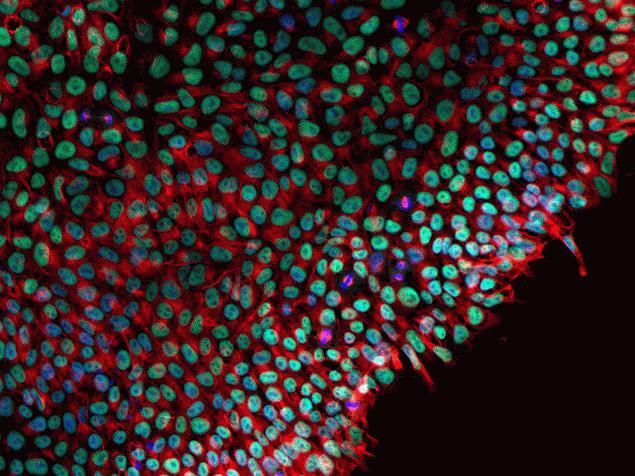
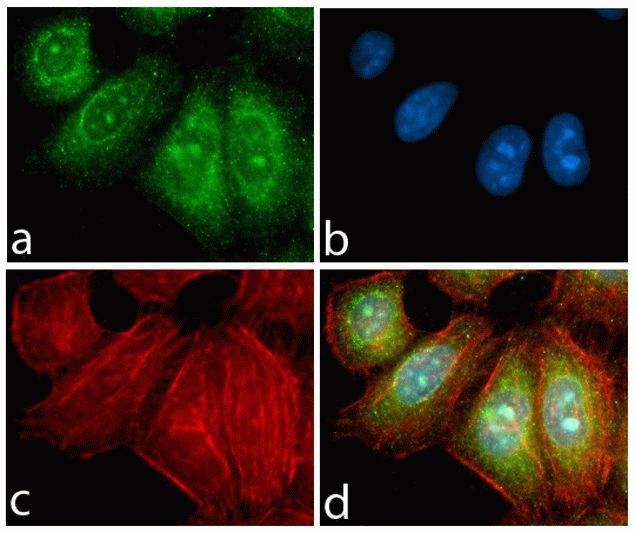
Phospho-FAK2 (Tyr579, Tyr580) Antibody (44-636G) in IF
Immunofluorescence analysis of Phospho-FAK2 / PYK2 pTyr580 was done on 70% confluent log phase A549 cells treated with 50ng of PDGF for 10 minutes. The cells were fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.1% Triton™ X-100 for 10 minutes, and blocked with 1% BSA for 1 hour at room temperature. The cells were labeled with Phospho-FAK2 / PYK2 pTyr580 Rabbit Polyclonal Antibody (44636G) at 2ug/ml in 0.1% BSA and incubated for 3 hours at room temperature and then labeled with Goat anti-Rabbit IgG (H+L) Superclonal™ Secondary Antibody, Alexa Fluor® 488 conjugate (Product # A27034) at a dilution of 1:2000 for 45 minutes at room temperature (Panel a: green). Nuclei (Panel b: blue) were stained with SlowFade® Gold Antifade Mountant with DAPI (S36938). F-actin (Panel c: red) was stained with Alexa Fluor® 555 Rhodamine Phalloidin (Product # R415, 1:300). Panel d is a merged image showing membranous localization. Panel e is untreated cell with no signal. Panel f is a no primary antibody control. The images were captured at 60X magnification.

F4/80 Antibody (MA5-16624) in IF
Immunofluorescent analysis of lymph node stained for macrophages using a F4/80 ANTIGEN monoclonal antibody (Product # MA5-16624) (conjugated to PE, red) and B cells using a CD79b monoclonal antibody (conjugated to a 488 fluorescent dye, green); nuclei are stained with DAPI, blue.

Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody (A32723) in IF
Immunofluorescent analysis of glial fibrillary acidic protein (GFAP) in E18 Sparague Dawley primary cortical neuronal cells containing astrocytes. The cells were fixed with 4% formaldehyde for 15 mins, permeabilized with 0.25% Triton X-100 in PBS for 10 mins, and blocked with 3% BSA in PBS for 30 mins at RT. Cells were stained with a GFAP mouse monoclonal antibody (Product # MA5-12023) at a dilution of 1:200 in 3% BSA in PBS for 1 hr at RT, and then incubated with Invitrogen Alexa Fluor Plus 488 goat anti-mouse IgG secondary antibody (Product # A32723) at a dilution of 1:1000 for 1 hr at RT. Nuclei were stained with Hoechst 33342 (product # H3570). The image contains overlay of GFAP (green) and nuclei (blue). Images were taken on a Zeiss LSM 710 confocal microscope at 40X magnification.
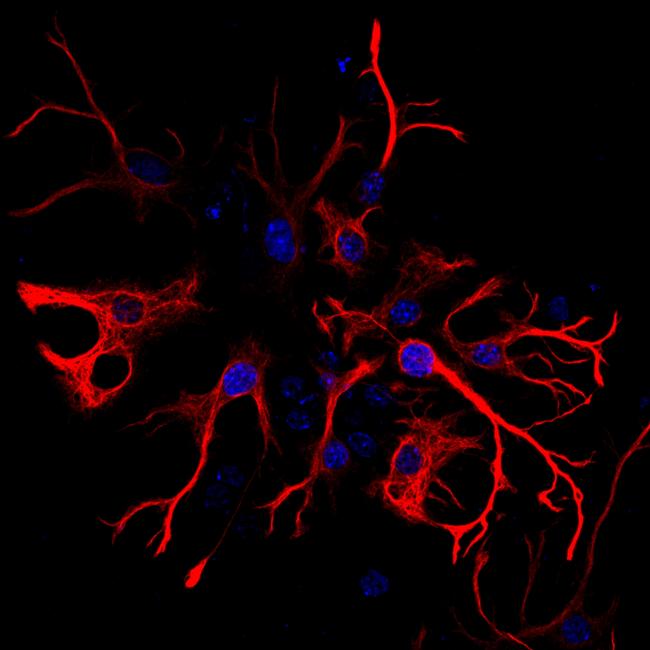
Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody (A32727) in IF
Immunofluorescent analysis of glial fibrillary acidic protein (GFAP) in E18 Sparague Dawley primary cortical neuronal cells containing astrocytes. The cells were fixed with 4% formaldehyde for 15 mins, permeabilized with 0.25% Triton X-100 in PBS for 10 mins, and blocked with 3% BSA in PBS for 30 mins at RT. Cells were stained with a GFAP mouse monoclonal antibody (Product # MA5-12023) at a dilution of 1:200 in 3% BSA in PBS for 1 hr at RT, and then incubated with Invitrogen Alexa Fluor Plus 555 goat anti-mouse IgG secondary antibody (Product # A32727) at a dilution of 1:1000 for 1 hr at RT. Nuclei were stained with Hoechst 33342 (product # H3570). The image contains overlay of GFAP (orange) and nuclei (blue). Images were taken on a Zeiss LSM 710 confocal microscope at 40X magnification.

Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody (A-11034) in IF
A 2.0 µm maize leaf section illustrating the immunolocalization of the enzyme ribulose bisphosphate carboxylase (rubisco) in the chloroplasts of the bundle sheath cells surrounding the vascular bundles. Maize is a C4 plant and, as a result, spatially segregates components of the photosynthetic process between the leaf mesophyll and the bundle sheath. Rubisco was localized using a rabbit anti-rubisco antibody and visualized using the highly cross-adsorbed Alexa Fluor® 488 goat anti–rabbit IgG antibody (Cat. No. A11034). The remaining fluorescence is due to the autofluorescence of chlorophyll, which appears red and is localized to the mesophyll plastids; lignin, which appears dull green and is localized to the xylem of the vascular bundle; and cutin, which appears bright green and is localized to the cuticle outside the epidermis. Image contributed by Todd Jones, DuPont.

Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody (A32728) in IF
Immunofluorescent analysis of β-III tubulin in E18 Sparague Dawley primary cortical neuronal cells. The cells were fixed with 4% formaldehyde for 15 mins, permeabilized with 0.25% Triton X-100 in PBS for 10 mins, and blocked with 3% BSA in PBS for 30 mins at RT. Cells were stained with a β-III tubulin mouse monoclonal antibody (Product # MA1-118) at a dilution of 1:200 in 3% BSA in PBS for 1 hr at RT, and then incubated with Invitrogen Alexa Fluor Plus 647 goat anti-mouse IgG secondary antibody (Product # A32728) at a dilution of 1:1000 for 1 hr at RT. Nuclei were stained with Hoechst 33342 (product # H3570). The image contains overlay of neuronal tubulin (far red) and nuclei (blue). Images were taken on a Zeiss LSM 710 confocal microscope at 40X magnification.

Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody (A32732) in IF
Immunofluorescent analysis of Grasp65 in A549 cells. The cells were fixed with 4% formaldehyde for 15 mins, permeabilized with 0.25% Triton X-100 in PBS for 10 mins, and blocked with 3% BSA in PBS for 30 mins at RT. Cells were stained with a Grasp65 rabbit polyclonal antibody (Product # PA3-910) at a dilution of 1:200 in 3% BSA in PBS for 1 hr at RT, and then incubated with Invitrogen Alexa Fluor Plus 555 goat anti-rabbit IgG secondary antibody (Product # A32732) at a dilution of 1:1000 for 1 hr at RT. Nuclei were stained with Hoechst 33342 (product # H3570). The image contains overlay of Grasp65(orange) and nuclei (blue). Images were taken on a Zeiss LSM 710 confocal microscope at 40X magnification.