产品介绍
产品名称: DiR(DiIC18(7)1,1′-dioctadecyltetramethylindotricarbocyanine Iodide)
分子式/分子量:C63H101IN2=1013.4
最大吸收波长/发射波长: 748/780 nm
推荐滤光片设置: 710 ex/760 em
结构式:
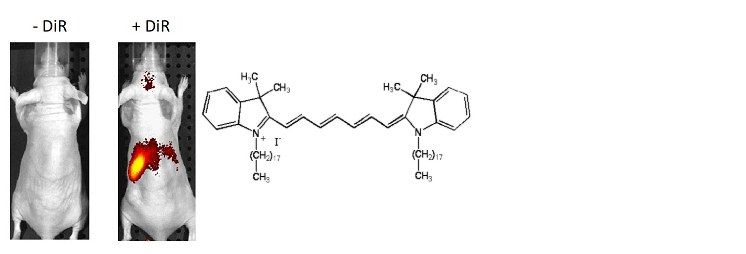
图1。从脾脏分离的T细胞用DiR和i.v进行荧光染色。(5×106个细胞/小鼠)注射到Nu / Nu小鼠中。上面用IVIS Spectrum注射后24小时拍摄的图像显示,细胞归巢于脾脏。
DiR染料是亲脂性的近红外花菁荧光染料,可以用来染细胞膜和其它脂溶性生物结构。18个碳的长链插入细胞膜中从而对细胞进行染色, 而细胞间的染料转移可以忽略不计。DiR的发射的是近红外荧光可以穿透细胞和组织,在活体成像中用来示踪。
DIR一般对原代细胞进行荧光染色并可进行活体成像分布观察, (例如下列细胞embryonic stem cells, bone marrow derived stem cells, adipose derived stem cells, lymphocytes and erythrocytes)

图2.通过将25 mg溶于3 mL乙醇来制备DiR储备液。通过在5 mL PBS中稀释199 µL储备液来制备320 µg / mL的工作溶液。 从脾脏分离的T细胞与320 µg / mL DiR孵育。 孵育30分钟后,将细胞在4ºC下以1000 rpm离心离心3分钟,得到蓝色沉淀。用PBS洗涤细胞两次并静脉内注射(5×106细胞/小鼠)。 对照组在PBS中注射5×106个细胞/小鼠。 在注射后10分钟,1小时,6小时和24小时用IVIS Spectrum对小鼠成像。 DiR成像的理想滤光片组是710 nm激发和760 nm发射。在所有时间点对小鼠的背侧和腹侧成像。注射后24小时,收集脑,骨头,脾,肝,肺和肾以用于离体成像。
非侵入性体内成像实时显示了向肝脏和脾脏注射的T细胞的归巢过程,这通过离体成像得到了证实。
参考文献:
Kalchenko et al.Use of lipophilicnear-infrared dye in whole-body optical imaging of hematopoietic cell homing. Journal of Biomedical optics, September/October 2006, Vol 11(5).
Product description
Product name: DiR(DiIC18(7)1,1′-dioctadecyltetramethylindotricarbocyanine Iodide)
Molecular formula/molecular weight: C63H101IN2=1013.4
Maximum absorption wavelength/emission wavelength: 748/780 nm
Recommended filter setting: 710 ex/760 em
Structural formula:
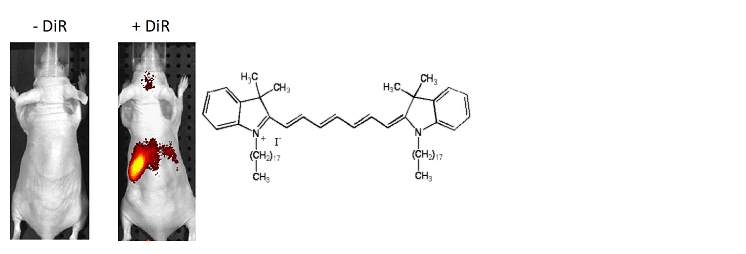
figure 1. T cells isolated from the spleen were fluorescently stained with DiR and i.v. (5×106 cells/mouse) were injected into Nu/Nu mice. The above image taken 24 hours after injection with IVIS Spectrum shows that the cells have homed to the spleen.
DiR dye is a lipophilic near-infrared cyanine fluorescent dye that can be used to dye cell membranes and other fat-soluble biological structures. A long chain of 18 carbons is inserted into the cell membrane to stain the cells, and the dye transfer between cells is negligible. DiR emits near-infrared fluorescence that can penetrate cells and tissues and is used for tracking in in vivo imaging.
DIR generally performs fluorescent staining of primary cells and can be used for in vivo imaging distribution observation, (for example, the following cells: embryonic stem cells, bone marrow derived stem cells, adipose derived stem cells, lymphocytes and erythrocytes)
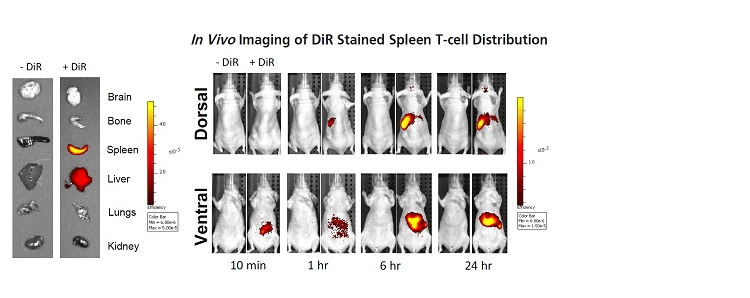
Figure 2. DiR stock solution was prepared by dissolving 25 mg in 3 mL ethanol. Prepare a working solution of 320 µg/mL by diluting 199 µL stock solution in 5 mL PBS. T cells isolated from the spleen were incubated with 320 µg/mL DiR. After incubating for 30 minutes, the cells were centrifuged at 1000 rpm at 4ºC for 3 minutes to obtain a blue precipitate. The cells were washed twice with PBS and injected intravenously (5×106 cells/mouse). The control group was injected with 5×106 cells/mouse in PBS. The mice were imaged with IVIS Spectrum at 10 minutes, 1 hour, 6 hours and 24 hours after injection. The ideal filter set for DiR imaging is 710 nm excitation and 760 nm emission. The dorsal and ventral sides of the mice were imaged at all time points. 24 hours after injection, brain, bone, spleen, liver, lung and kidney were collected for ex vivo imaging.
Non-invasive in vivo imaging shows the homing process of T cells injected into the liver and spleen in real time, which is confirmed by ex vivo imaging.
References:
Kalchenko et al.Use of lipophilicnear-infrared dye in whole-body optical imaging of hematopoietic cell homing. Journal of Biomedical optics, September/October 2006, Vol 11(5).