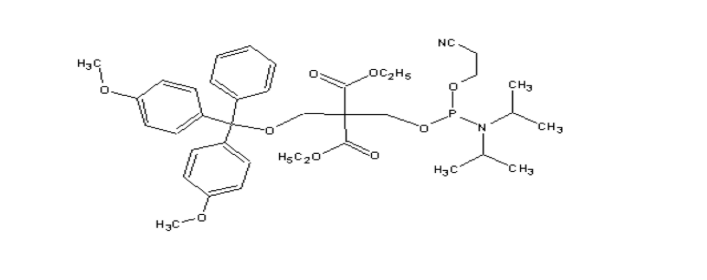
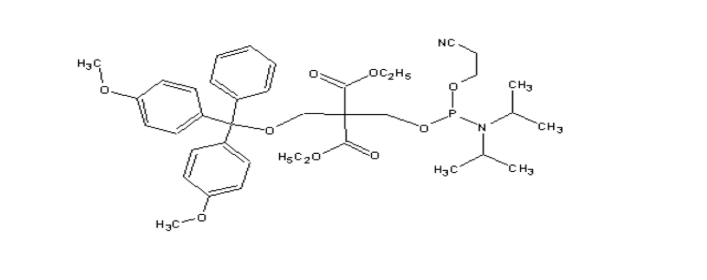
Chemical Phosphorylation Reagent I (CPR I)
基本信息
| 产品名称 | Chemical Phosphorylation Reagent I (CPR I) |
|---|---|
| 英文名称 | Chemical Phosphorylation Reagent I (CPR I) |
| 运输条件 | 超低温冰袋运输 |
一般描述
Product description
Solvent:MeCN,DMSO
MW : 656.77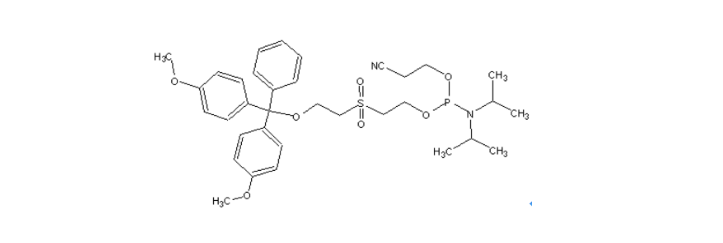
Features and Biological Applications
This reagent can be used to create the 3′-phosphate by adding it as the first addition to the support in Labeling Oligonucleotides with Fluorescent Dyes. After ammonia deprotection the oligo will have the 3′-phosphate attached to the 2nd base added during synthesis. Both the support base and the CPR are cleaved. The DMT-group is removed during the ammonium hydroxide deprotection and thus is not available for poly-pak purification.
References
1. Xu Y, Lee SA, Kutateladze TG, Sbrissa D, Shisheva A, Prestwich GD. (2006) Chemical synthesis and molecular recognition of phosphatase-resistant analogues of phosphatidylinositol-3-phosphate. J Am Chem Soc, 128, 885.
2. Ohkubo A, Ezawa Y, Seio K, Sekine M. (2004) O-selectivity and utility of phosphorylation mediated by phosphite triester intermediates in the N-unprotected phosphoramidite method. J Am Chem Soc, 126, 10884.
3. Tsuruoka H, Shohda K, Wada T, Sekine M. (2000) Synthesis and conformational properties of oligonucleotides incorporating 2′-O-phosphorylated ribonucleotides as structural motifs of pre-tRNA splicing intermediates. J Org Chem, 65, 7479.
4. Olejnik J, Krzymanska-Olejnik E, Rothschild KJ. (1996) Photocleavable biotin phosphoramidite for 5′-end-labeling, affinity purification and phosphorylation of synthetic oligonucleotides. Nucleic Acids Res, 24, 361.
5. Mora N, Lacombe JM, Pavia AA. (1995) A new approach to phosphoserine, phosphothreonine and phosphotyrosine synthons and to thiophospho analogs. Stepwise synthesis of mono- and multiphosphorylated phosphopeptides related to src-protein kinase. Int J Pept Protein Res, 45, 53.
6. Boumendjel A, Miller SP. (1994) Synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. J Lipid Res, 35, 2305.
7. Kitas E, Kung E, Bannwarth W. (1994) Chemical synthesis of O-thiophosphotyrosyl peptides. Int J Pept Protein Res, 43, 146.
8. Tegge W, Ballou CE. (1992) Syntheses of D-myo-inositol 1,4,5-trisphosphate affinity ligands. Carbohydr Res, 230, 63.
9. Perich JW, Reynolds EC. (1991) Fmoc/solid-phase synthesis of Tyr(P)-containing peptides through t-butyl phosphate protection. Int J Pept Protein Res, 37, 572.
10. Lacombe JM, Andriamanampisoa F, Pavia AA. (1990) Solid-phase synthesis of peptides containing phosphoserine using phosphate tert.-butyl protecting group. Int J Pept Protein Res, 36, 275.
Product description
Solvent:MeCN,DMSO
MW : 656.77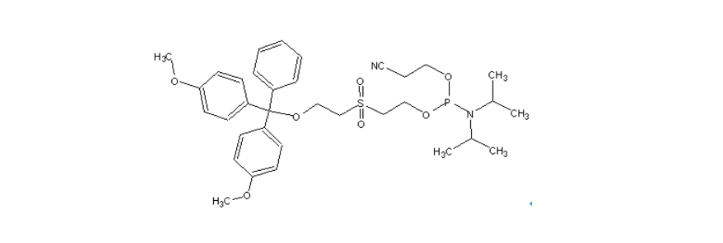
Features and Biological Applications
This reagent can be used to create the 3′-phosphate by adding it as the first addition to the support in Labeling Oligonucleotides with Fluorescent Dyes. After ammonia deprotection the oligo will have the 3′-phosphate attached to the 2nd base added during synthesis. Both the support base and the CPR are cleaved. The DMT-group is removed during the ammonium hydroxide deprotection and thus is not available for poly-pak purification.
References
1. Xu Y, Lee SA, Kutateladze TG, Sbrissa D, Shisheva A, Prestwich GD. (2006) Chemical synthesis and molecular recognition of phosphatase-resistant analogues of phosphatidylinositol-3-phosphate. J Am Chem Soc, 128, 885.
2. Ohkubo A, Ezawa Y, Seio K, Sekine M. (2004) O-selectivity and utility of phosphorylation mediated by phosphite triester intermediates in the N-unprotected phosphoramidite method. J Am Chem Soc, 126, 10884.
3. Tsuruoka H, Shohda K, Wada T, Sekine M. (2000) Synthesis and conformational properties of oligonucleotides incorporating 2′-O-phosphorylated ribonucleotides as structural motifs of pre-tRNA splicing intermediates. J Org Chem, 65, 7479.
4. Olejnik J, Krzymanska-Olejnik E, Rothschild KJ. (1996) Photocleavable biotin phosphoramidite for 5′-end-labeling, affinity purification and phosphorylation of synthetic oligonucleotides. Nucleic Acids Res, 24, 361.
5. Mora N, Lacombe JM, Pavia AA. (1995) A new approach to phosphoserine, phosphothreonine and phosphotyrosine synthons and to thiophospho analogs. Stepwise synthesis of mono- and multiphosphorylated phosphopeptides related to src-protein kinase. Int J Pept Protein Res, 45, 53.
6. Boumendjel A, Miller SP. (1994) Synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. J Lipid Res, 35, 2305.
7. Kitas E, Kung E, Bannwarth W. (1994) Chemical synthesis of O-thiophosphotyrosyl peptides. Int J Pept Protein Res, 43, 146.
8. Tegge W, Ballou CE. (1992) Syntheses of D-myo-inositol 1,4,5-trisphosphate affinity ligands. Carbohydr Res, 230, 63.
9. Perich JW, Reynolds EC. (1991) Fmoc/solid-phase synthesis of Tyr(P)-containing peptides through t-butyl phosphate protection. Int J Pept Protein Res, 37, 572.
10. Lacombe JM, Andriamanampisoa F, Pavia AA. (1990) Solid-phase synthesis of peptides containing phosphoserine using phosphate tert.-butyl protecting group. Int J Pept Protein Res, 36, 275.
相关属性
| 敏感性 | 对光和湿度敏感 |
|---|---|
| 储存温度 | 避光,-20°C储存,干燥 |
| 品牌 | Jinpan |